FAQs
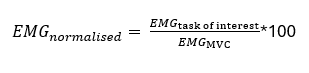
Normalisation is necessary to reduce the impact of differences in physiological and anatomical characteristics of muscles and surrounding tissues (Besomi et al., 2020).
There are different scenarios where non-normalizing EMG amplitude data can be acceptable. However, this depends on the research question/outcome. For example, when you are comparing the EMG amplitude within a person and muscle, between conditions/tasks (within a session, i.e., without removing electrodes). Answer the questions in the decision-support tool to find out more: Access the decision-support tool.
Applying identical data processing procedures (e.g., filtering) to both task-related recordings and the reference data used for normalization (e.g., MVCs) is a necessary step toward achieving high-quality EMG amplitude data, although additional checks are required to identify potential artefacts and ensure robustness. In addition, visual inspection is a critical practice for detecting artefacts that may not be removed through filtering alone (Boyer et al., 2023). This is particularly relevant during dynamic contractions, which are more susceptible to movement-related noise. Visual inspection should always be performed on spatially filtered signals (at least single differential), rather than monopolar signals, to improve artefact detection and interpretation. Filtering can sometimes make an artefact resemble EMG, so reviewing both filtered and unfiltered signals simultaneously can help detect such issues. When using high-density surface EMG (HDsEMG), visual inspection becomes especially important for identifying "bad channels" that could introduce noise into the signal (Del Vecchio et al., 2020). In this context, inspection should be conducted after computing a single-differential derivation. At present, no automated signal processing methods reliably detect bad channels, making visual inspection a necessary part of the quality control process (Gallina et al., 2020). It is also advisable to assess baseline noise levels. While a perfectly noise-free baseline may be unrealistic, aiming for a signal-to-noise ratio (SNR) of at least 100 between MVC and rest conditions can help reduce error, particularly when interpreting low-level muscle activity. An alternative approach is to subtract the baseline noise from both the task under investigation and the MVC. While these steps are essential to support valid normalization, they do not guarantee data quality in all cases. Researchers must apply informed judgment when evaluating signal quality, as undetected artefacts may still persist despite adherence to best-practice procedures.
The CEDE amplitude normalization matrix includes a range of commonly used EMG amplitude normalization approaches. However, these methods are not equally valid or appropriate for all contexts. Other methods included in the matrix are:
- Normalization to a standardized isometric MVC, which may be used when the task of interest is complex or involves multiple muscles or task types;
- Normalization to a standardized submaximal task (isometric or dynamic) with similar characteristics to the task of interest;
- Normalization to the peak or mean EMG amplitude during the task investigated;
- Non-normalized EMG amplitude.
- Normalization to maximal M-wave amplitude, only appropriate for normalization of evoked responses.
To determine which method is most appropriate for your specific research context, use the decision-support tool provided in the CEDE matrix.
Where feasible, normalization to the maximum voluntary contraction (MVC) performed in the same context as the task of interest (i.e., matched contraction type, muscle length/joint angle, and/or velocity) is generally preferred, as it provides the most physiologically meaningful comparison.
A reference value is the value used for normalization. It serves as the baseline against which EMG amplitude data are compared. EMG amplitude, force, or torque values will be expressed as a percentage of the reference value. For more information about normalization,click here.
Muscle activation refers to the number of motor units recruited and their firing rates (i.e., the number of muscle fibers activated and the firing rates at which they are activated) (McManus et al., 2021).
There is no quantitative measurement of muscle activation, but EMG amplitude is often used as an indirect estimate, as it reflects the electrical activity generated by muscle fibers. However, EMG amplitude is influenced by multiple factors related to activation (e.g., motor unit recruitment and firing rate), and the relationship is not necessarily linear.
The interference EMG is the summation of action potentials from active motor units and appears as a noise-like waveform. The intensity of this signal is commonly estimated using measures such as: Average Rectified Value (ARV) and Root Mean Square (RMS) amplitude. This involves rectifying the raw (interference) EMG signal and then low-pass filtering the rectified signal to smooth out the random fluctuations.
The EMG amplitude estimates the intensity of the raw EMG (expressed as averaged-rectified value [ARV] or root-mean-square [RMS]) signal at each instant in time.
While EMG amplitude is frequently used as a surrogate for muscle activation, it should be interpreted with caution, as the relationship between EMG amplitude and activation can be task-dependent and nonlinear (e.g., due to factors like muscle fatigue, electrode placement, and crosstalk) (Clancy et al., 2023).
A muscle can be considered relaxed when there is no detectable action potential from its motor units. When surface electrodes are placed parallel to the muscle fascicles direction, this can often be assessed by identifying periods of minimal EMG amplitude and the absence of action potential signals.
The most confident surface-based approach involves using a matrix (array) of surface electrodes to detect spatial propagation across adjacent electrodes (with at least one electrode row positioned along the fiber direction). There are some issues to consider. This approach will; (1) only identify activity of relatively superficial motor units and not deeper fascicles, (2) may be less sensitive in pennate muscles, and (3) because of crosstalk from adjacent muscles, some EMG activity might still be recorded even if the muscle of interest is relaxed. Greater confidence in excluding crosstalk and detect activation from deeper fascicles would be provided by intramuscular recordings.
A contraction type refers to how a muscle generates force relative to the load and joint movement (Hill, 1925). These are typically classified based on the behavior of the muscle–tendon unit, but more precisely reflect changes in muscle fiber length, which can differ due to tendon compliance.
The three main types of muscle contractions are:
- Isometric contraction: The muscle generates force without a change in joint angle. However, due to tendon stretch, muscle fibers may still shorten slightly even when the joint position is held constant (e.g., holding a weight in a fixed position).
- Concentric contraction: The muscle fibers shorten while producing force, typically to overcome a load (e.g., lifting a dumbbell in a bicep curl).
- Eccentric contraction: The muscle fibers lengthen while producing force, typically as they resist an external load (e.g., lowering a dumbbell in a bicep curl).
These classifications are helpful for basic understanding but can become ambiguous during complex, dynamic tasks where load and movement vary over time. In these contexts, terms like "dynamic contraction" (contractions involving joint movement) and "force-varying contraction" (where muscle force changes continuously) may be more appropriate.
The force-velocity relation describes how the amount of force a muscle can produce varies with the velocity of contraction (i.e., how fast the muscle is shortening or lengthening) (Alcazar et al., 2019).
- During concentric contractions (when the muscle shortens), the faster the contraction velocity, the less force the muscle can produce.
- During eccentric contractions (when the muscle lengthens), the faster the muscle is lengthened, the more force it can resist or produce.
- At zero contraction velocity, the contraction is isometric, and force production is near maximal but without fiber length change.
This relationship applies at the muscle fiber level, though in practice, contraction velocity is often approximated from joint angular velocity, which is not always equivalent.
The force–velocity relationship is classically represented as a curve with three regions:
- Positive velocities: concentric (shortening)
- Zero velocity: isometric
- Negative velocities: eccentric (lengthening)
This foundational principle of muscle physiology was first described by Hill (1938), and remains central to understanding how muscles generate and resist force under different movement conditions.
Muscle fatigue is typically defined as a reduction in the muscle’s ability to produce force. The gold standard for assessing fatigue is to directly measure changes in force output under controlled conditions (Enoka et al., 2008).
However, surface EMG analysis can provide indirect information about neuromuscular fatigability—that is, the muscle's susceptibility to fatigue under a given task (Sun et al., 2022).
When a muscle is fatiguing (especially during sustained isometric contractions), two EMG-based features are commonly observed:
- A decrease in median frequency of the EMG power spectrum
- A decrease in muscle fibre conduction velocity
- An increase in EMG amplitude (due to increased motor unit recruitment and firing rate)
These changes reflect adaptations in motor unit behavior, but they are not definitive markers of fatigue. Interpretation should consider task type, muscle architecture, electrode setup, and the potential for compensation by other muscles.
Importantly, EMG features can vary substantially between individuals and tasks, and should not be used as standalone indicators of fatigue. Whenever possible, EMG analysis should be combined with force measurements for a more complete understanding of fatigue development.
To validly compare EMG amplitude between individuals or groups, it is essential to use a normalization procedure. Without normalization, differences in EMG amplitude may simply reflect non-physiological factors such as electrode placement, skin impedance, or subcutaneous fat, rather than actual differences in muscle activation.
The most commonly recommended approach is to normalize EMG amplitude to a reference value (e.g., a maximal contraction), ideally matched in contraction type, joint angle, and velocity to the task of interest.
For more information on which method is most appropriate for your study context, use our decision-support tool here.
Click here to learn more about how to properly normalize the EMG amplitude data in order to:
- Evaluate the effect of an intervention (pre–post intervention design) within a single session.
- Evaluate the effect of an intervention (pre–post intervention design) longitudinally (between sessions).
Click here to learn more about how to properly normalize the EMG amplitude data to compare different conditions.
It is strongly recommended to plan for EMG amplitude normalization during the study design phase, as this affects key elements of the protocol (e.g., whether to perform MVCs).
However, if normalization was not considered initially, it may still be possible to apply it retrospectively depending on your research question and the data collected. Some approaches—such as within-task normalization or task-specific reference values—may still be appropriate.
Use the decision-support tool to explore which normalization options are feasible and justifiable based on your specific context.
One way to verify whether a voluntary contraction has been performed at the participant’s maximal capacity is to apply the twitch interpolation technique, where an electrical stimulus is applied during the contraction. If additional force is elicited, it suggests that the muscle was not fully activated voluntarily. However, this method may not be practical or available in all settings, and it is important to consider specific conditions in order to ensure that the technique is reliable (Allen et al., 1995) .
- Observation of effort and technique – Look for clear physical signs of exertion and provide strong verbal encouragement to promote maximal voluntary performance.
- Participant feedback – Ask whether the participant felt they gave their maximal effort during the task.
- Performance consistency – Large variability across MVC trials may indicate that the participant did not fully understand the task or failed to exert consistent effort.
- Normalized EMG review – If EMG signals collected during a task exceed those recorded during an MVC (i.e., >100% normalized), it may indicate the MVC was underperformed. However, this is less reliable for fast dynamic tasks, where EMG can naturally exceed isometric MVC values due to different recruitment strategies.
It is important to consider that even with maximal intent, actual muscle activation levels can vary depending on factors like joint angle, task familiarity, and neural drive.
It is generally suggested to perform 3 to 5 repetitions of an MVC, with an adequate amount of rest between repetitions (Click here to know more about rest).
To minimize a reduction in force capacity and ensure each contraction reflects true maximal effort, it is generally recommended to allow a rest period of at least 1 minute between MVCs. If the task involves multiple muscle groups, sustained high effort, or if there are signs of inconsistent performance, consider extending the rest period to 3–5 minutes to allow for full recovery (Freitas de Salles, 2012); (Cheng-Lung et al., 2022).
The optimal duration may depend on the muscle group, contraction duration, and participant fatigue status. Consistency in MVC EMG or force across repetitions is a useful indicator that rest periods are sufficient.
Whether this reflects an underperformed MVC or a genuine difference in task demands depends on when and how the issue is identified:
During data collection:
- If the participant is still available, consider repeating the MVC trial after providing:
- Adequate rest to minimize fatigue
- Strong verbal encouragement and visual feedback of force output, when possible, which is more effective than verbal cues alone
- Additional support to ensure the participant understands the task and target intensity
During data processing:
- If the task-related force or EMG amplitude exceeds that of the MVC, it may suggest:
- The MVC was underperformed, or
- The task context (e.g., joint angle, velocity, or movement type) allowed for greater force or activation than the MVC setup
In such cases:
- Evaluate whether the task legitimately allows for greater output (e.g., due to differences in joint angle, velocity, or fascicle length). For instance, muscles may generate more force in the task of interest than in the MVC task if the task places them at a more optimal position on the length–tension curve
- If the task and MVC context differ, this potential for greater force or EMG output must be considered when designing and interpreting MVC-based normalization
- If the discrepancy likely reflects an underperformed MVC, and no additional MVC trial is available, the affected data may need to be excluded from analysis
- When traditional MVC-based normalization is not suitable, consider alternative methods, such as peak amplitude during the task, only if the research aim allows for such methods (see our decision-support tool to find your best solution)
Note: It’s not uncommon for EMG amplitude during dynamic tasks to exceed that of an isometric MVC due to differences in motor unit recruitment strategies. This does not necessarily invalidate the data—but it must be clearly interpreted and reported.
In laboratory settings, an MVC is typically performed against a rigid restraint or other controlled resistance devices, which ensures consistency and standardization across trials. However, these setups are often impractical in field settings.
For field-based MVC assessments, the following alternatives can be considered (for isometric contractions only):
- Manual resistance applied by a trained examiner.
- Use of straps, belts, or bands to provide stable resistance.
- Hand-held dynamometers or portable load cells can be used to provide an objective measure of the force produced, when the force is applied in line with the sensor axis. However, these tools quantify the effort generated and do not confirm whether a true MVC has been achieved.
Important considerations:
- Reliability and consistency: Manual resistance depends heavily on the examiner’s strength, technique, and consistency. It is less reliable across sessions or examiners.
- Applicability: These methods are appropriate only for isometric contractions. Isotonic or dynamic MVCs typically require external stabilization that is hard to replicate outside of a lab.
- Reporting: Any deviations from standard MVC procedures—such as using manual or field-based setups—should be clearly described and justified in your methodology section.
- Measurement limitations: Load cells and force transducers can help objectively quantify the effort produced during a contraction, but they may not represent a true MVC without proper standardization and stabilization.
Replacing electrodes during data collection is a major issue, as it can introduce significant variability in EMG signal amplitude and reliability. Below are key considerations and recommended actions:
- If Maximum Voluntary Contraction (MVC) was performed: MVC should be repeated after electrode replacement to ensure accurate normalization.
- Data Compatibility: Pre- vs. Post-electrode replacement.
- Caution is required when analyzing and interpreting data before and after electrode replacement.
- Differences in signal amplitude may not reflect physiological changes but rather artefacts caused by electrode repositioning.
- To minimize variability, standardized and repeatable protocols for electrode placement should be followed.
- Marking electrode positions on the skin before replacement can help maintain consistency.
- Ideal approach: Data recollection.
- Whenever possible, recollecting the data (after providing a break) is the best approach to ensure consistency and data integrity.
- If recollection is not feasible, carefully document the replacement process and record any observed changes in signal quality to account for potential errors in the analysis.
The twitch interpolation technique is used to assess how completely a person can voluntarily activate a muscle during a maximal contraction. It involves delivering a brief electrical stimulus to a motor nerve during a maximal voluntary contraction (MVC). If the muscle is not fully activated, the stimulus produces an additional "twitch-like" increase in force, indicating that some motor units were not voluntarily recruited (Herbert & Gandevia, 1999).
While widely used in research and clinical settings, it is not feasible in all muscles, particularly:
- Muscles that are deep or have multiple innervating nerves,
- Muscles innervated by nerves not easily accessible to stimulation,
- Muscles that cannot be stimulated safely, or
- Contexts where force output is not easily measured (Shield & Zhou, 2004).
In addition, interpretation can be complicated by factors such as:
- Additional force generation from muscles innervated by the same nerve,
- Unintended activation of antagonist muscles,
- Fatigue,
- High tendon compliance, and
- Non-linear twitch-force relationships at submaximal intensities (Behm et al., 1996; Gandevia, 2001).
As such, although informative, the technique can be difficult to perform reliably and should be applied with careful methodological controls.
A motor-evoked potential (MEP) is the electrical response recorded from a muscle following stimulation of the motor cortex, typically using transcranial magnetic stimulation (TMS) (Rossini et al., 1994). The MEP reflects the compound muscle action potential elicited by descending motor signals and is commonly recorded using surface EMG.
MEPs are used to assess the excitability of the corticospinal pathway, and they are distinct from the mechanical twitch response (i.e., force) that may also follow cortical or peripheral stimulation.
Note: Although TMS is the most common technique, MEPs can also be elicited using electrical stimulation of the cortex in some research or clinical settings.
The H-reflex (or Hoffmann reflex, 1910) is a spinal reflex elicited by electrically stimulating a peripheral motor nerve, typically through a mixed nerve containing both sensory and motor fibers (Palmieri et al., 2004).
Unlike the stretch reflex, which is triggered by mechanical stimulation of muscle spindles, the H-reflex bypasses the muscle spindle and directly activates Ia afferent fibers. This leads to a monosynaptic reflex arc, resulting in a muscle response that is recorded via surface EMG. Although the H-reflex activates part of the same spinal circuitry as the stretch reflex, it is not dependent on gamma motor activity and should not be considered equivalent.
The H-reflex is commonly used to assess spinal excitability and neuromuscular function in both clinical and research settings.
An M-wave, also known as a compound muscle action potential (CMAP), is an electrical response recorded from a muscle following direct electrical stimulation of a motor nerve or, in some cases, the muscle itself (stimulating the motor axons) (Palmieri et al., 2004).
It reflects the synchronous activation of multiple motor units and is recorded using surface EMG. The M-wave is commonly used to assess neuromuscular transmission, peripheral nerve excitability, and to monitor muscle response during reflex or voluntary testing.
Note: While a mechanical twitch may accompany the stimulation, the M-wave refers specifically to the electrical potential, not the force output.
The H-reflex and M-wave are both muscle responses elicited by applying electrical stimulation to a mixed peripheral nerve (e.g. the tibial nerve, which contains both Ia afferents and motor efferent fibers). However, both responses represent different physiological mechanisms and require different stimulation intensities.
- The H-reflex is a response that reflects spinal sensorimotor excitability. Compared to similar sized M-waves, it is best elicited by lower-intensity stimulation of the mixed nerve. Lower intensities preferentially activate Ia afferents due to their larger diameter and lower threshold for excitation, causing the Ia afferents to send action potentials to the spinal cord. From this, the Ia afferents recruit alpha motor neurons, that send action potentials to the muscle via their axons — activating the same monosynaptic reflex pathway used in the stretch reflex.
- The M-wave, on the other hand, is a response that reflects excitability of the motor axon or muscle. Compared to similar sized H-reflexes, it is elicited by higher-intensity stimulation of the mixed nerve. Higher intensities directly depolarize motor axons, causing a shorter latency muscle response that bypasses the spinal cord. The resulting EMG response reflects direct activation of the motor axons and innervating muscle fibres.
These responses are often recorded together in electrophysiological studies to assess neuromuscular excitability and spinal vs. peripheral contributions to muscle activation.
Each of these represents an electrically recorded muscle response (with surface EMG), but they differ in how the nervous system is stimulated and which part of the neuromuscular pathway is assessed:
- M-wave: Elicited by direct electrical stimulation of motor axons in a peripheral nerve. It reflects direct activation of the muscle without spinal involvement.
- H-reflex: Elicited by electrical stimulation of Ia afferent fibers in a peripheral nerve. The signal travels to the spinal cord and then returns to the muscle via alpha motor neurons—activating a monosynaptic reflex loop.
- MEP (motor-evoked potential): Elicited by stimulating the motor cortex, typically using transcranial magnetic stimulation (TMS). The signal travels through central descending pathways to reach the motor neurons and then the muscle.
While all three produce measurable EMG responses, they probe different components of the nervous system—from the cortex (MEP) to the spinal cord (H-reflex), to the peripheral nerve (M-wave) (Theodosiadou et al., 2023).
In the context of the “Amplitude Normalization Matrix” (Besomi et al., 2020), a within-muscle/person/group comparison (also known as a within-participant design) is a study design where all muscles or participants are exposed to the same treatment, intervention, or experimental conditions.
The primary goal is to measure changes in the outcome of interest over time or between conditions within the same subjects, rather than comparing different groups.
Key characteristics:
- Each participant/muscle/group serves as its own control, reducing variability and increasing statistical power.
- Repeated measurements are taken under different conditions or at different time points.
- Differences are assessed within the same entity, rather than between separate groups.
Examples:
- In EMG studies, within-muscle designs might compare the same muscle's activation before and after an intervention, or between different muscles within the same subject.
- In rehabilitation research, a within-person study could track pain levels in the same individual before and after a physiotherapy program.
- In exercise science, a within-group design could examine how a group of runners adapts to a new training regimen over time.
In the context of the “Amplitude Normalization Matrix” (Besomi et al., 2020), between-session reproducibility (also known as repeatability) refers to the degree of consistency in measurements when the same procedure is repeated under identical conditions on different days or separate sessions.
It assesses whether the same outcome is obtained when the measurement is performed at different time points, ensuring reproducibility over time and reflects the potential error or difference in an outcome that is due to time/measures and not the intervention (Bland & Altman, 1986).
We value your feedback
Let us know how helpful you found the recommendations above and how we can improve: